

iMediSync is attracting a great attention at CES 2021 with its novel headset, iSyncWave™. It was introduced as one of the Hot Products by the Consumer Electronics Show organizer.
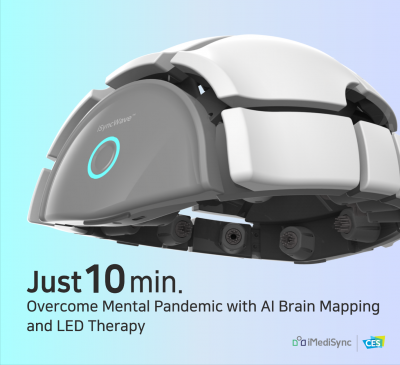
iSyncWave™ is AI brain Mapping and personalized NIR-LED photobiomodulation therapy that will enhance neural activity; optimizing a person’s brain.
iMediSync claims they will tack the current mental pandemic and Corona Blue with its novel approach to biomarker development and personalized photobiomodulation therapy. This will accessible not only at your doctor’s clinic, but at the comfort of your own home.
iMediSync is a leading company in an EEG brain mapping market that has currently tackled Alzheimer’s. Through their iSyncBrain® AI driven cloud platform, they developed a biomarker and are able to screen with more than 90% accuracy the likelihood of a person progressing into full-blown Alzheimer’s up to 10 years before a conventional diagnosis.
CES 2021 is ALL-DIGITAL for the first time in its history. The show will ends at 8 PM on January 13th. You can set up a meeting with iMedISync at the following link:
https://digital.ces.tech/exhibitor/5a85273d-ece3-4d37-ad38-7ab3b4c66f50?source=exhibitors
About CES
CES® is the most influential tech event in the world —proving the ground for breakthrough technologies and global innovators. This is where the world's biggest brands do business and meet new partners, and the sharpest innovators hit the stage. Owned and produced by the Consumer Technology Association (CTA)®, CES features every aspect of the tech sector.
About iMediSync
iMediSync (virtual.imedisync.com) has developed an AI-driven early detection and therapeutic platform for optimal brain health. iMediSync launched its first solution to detect Mild Cognitive Impairment (early stage of Alzheimer’s Disease) based on its proprietary EEG database in Korea last August 2020 (iSyncBrain MCI Classifier – KFDA Cleared). They conducted the clinical trial with multicenter hospitals with the result of 90%+ accuracy for its solution. The vision of iMediSync, just like iSyncBrain MCI Classifier, is to develop early detecting biomarkers for challenging neurological disorders and diseases, such as COMA, Parkinson’s, ADHD, Depression and etc.
Contact
Joshua Choi, General Manager of Sales & Marketing, iMediSync, Inc.
actsjung@imedisync.com
Jane Han, Manager of Sales & Marketing, iMediSync, Inc.
janehan@imedisync.com